
Данный сайт доступен только для медицинских работников.
Вы являетесь специалистом в сфере здравоохранения?

The only domestic medication of a pulmonary surfactant
The medication is included in the Temporary Methodological Recommendations
“Prevention, diagnosis and treatment of the novel coronavirus infection”
of the Ministry of Health of the Russian Federation


Surfactant-BL is developed for the treatment of respiratory distress syndrome (RDS) in newborns. The cause of this disease is the lung surfactant deficiency associated with the immaturity of the lung tissues of a premature newborn.
Adult respiratory distress syndrome is also associated with damage to the pulmonary surfactant system.
In 2021, it was included in the Temporary Methodological Recommendations “Prevention, diagnosis and treatment of the novel coronavirus infection” of the Ministry of Health of the Russian Federation.
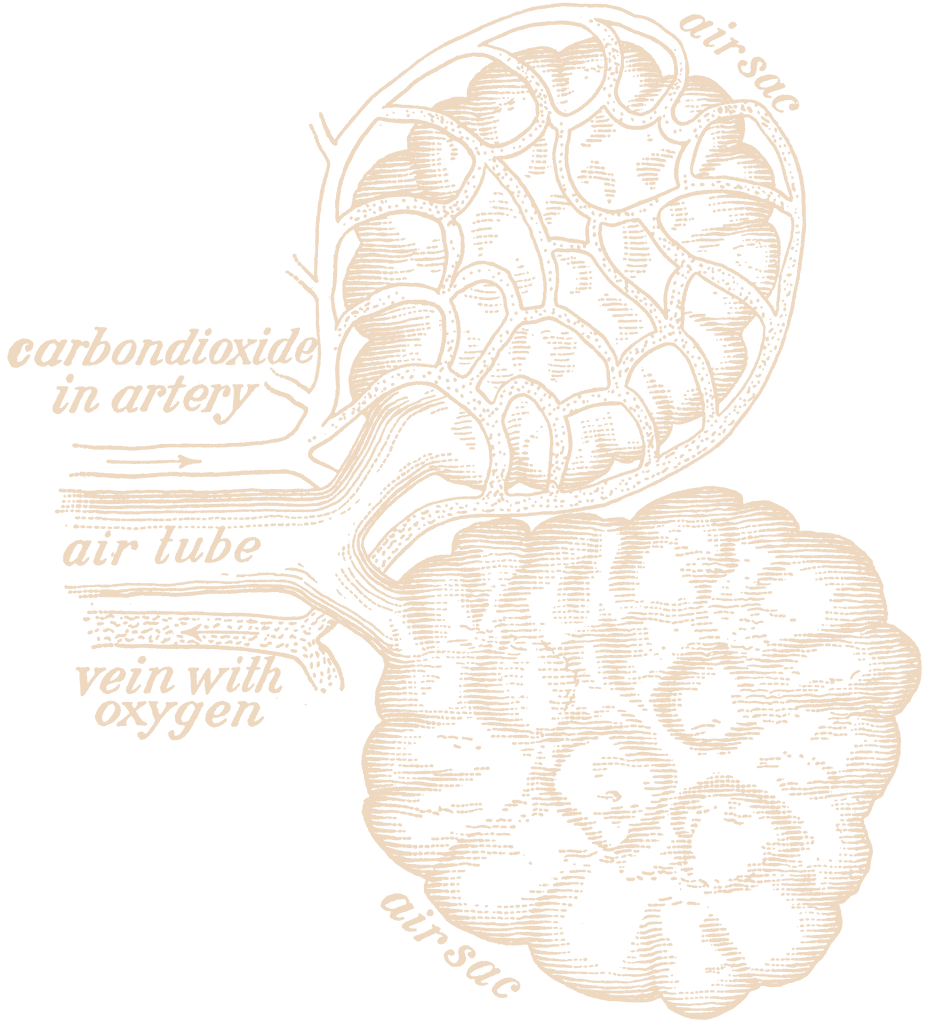
Pulmonary surfactant – is a multicomponent lipoprotein complex that performs a number of important functions in the body.
The composition of Surfactant-BL is as close to endogenous as possible, which explains its effectiveness in diseases associated with a deficiency or change in the composition of the pulmonary surfactant.
Other medications of lung surfactants are poorer in components, since important surfactant-associated proteins are removed during their production. Along with the loss of complexity of components, a number of important functions are also lost, which explains the need for a higher therapeutic dose and the lack of effect in adults, in contrast to Surfactant-BL.
The composition of modern synthetic medications includes surfactant-associated proteins obtained by genetic engineering. But due to the simplified composition, these drugs are not able to fully replace the endogenous surfactant.
~200,000 cases of acute respiratory distress syndrome (ARDS) in adults are diagnosed worldwide annually.
Mortality in ARDS
However, none of the world-marketed pulmonary surfactants have been able to obtain approval for ARDS therapy.
The experience of its use in more than 7,000 patients with ARDS has demonstrated that its use, during the first day (preferably hours) of the development of severe hypoxemia, during comprehensive basic therapy and competent lung ventilation allows:

1 Quickly leave damaging modes in lung ventilation.

2 Significantly (by 28-30 hours compared to the control) reduce the time needed to reach a non-toxic oxygen concentration (40%) in the inhaled gas mixture.

3 Reduce the time spent by newborns with lung ventilation up to 2-8 days.
4 Significantly reduce the frequency of complications of lung ventilation and of the neonatal period, such as intraventricular hemorrhages and bronchopulmonary dysplasia.

5 Significantly (by 2-3 times) reduce the rate of mortality from respiratory distress syndrome.
The study is based on data from 140 patients affected by mycobacteria which show multidrug resistance. All patients were persons discharging bacteria, treated for 2-6 months without positive dynamics..
It is shown that an 8-week course of administration (600-700 mg) of Surfactant-BL in combination with 4-5 anti-tuberculosis drugs allows to achieve:
Elimination of bacilli (disappearance of bacteria in sputum)
Resolution of infiltrate
Closure of necrotic cavities
In Russia, during the 2009-2010 A/H1N1 influenza epidemic.
more than 200 patients diagnosed with bilateral viral pneumonia were treated with Surfactant-BL in combination with antiviral therapy.
Positive results were obtained when Surfactant-BL was used in a complex therapy of severe pneumonia in patients with the COVID-19 infection.
In most cases, early inclusion of the Surfactant-BL medication in the treatment regimen of pneumonia by inhalation through a nebulizer at a dosage of 75 mg twice a day prevents the development of ARDS and putting patients on a ventilator.
In already developed ARDS, the use of Surfactant-BL along with antiviral and respiratory therapy shortens breathing support, relieves ventilation parameters, reduces mortality and prevents complications caused by pulmonary fibrosis.
Lung surfactant medications have been successfully used in Russia by fire victims for the purpose of prevention and treatment of ARDS in inhalation trauma and burn disease.
The mortality rate in patients receiving treatment with Surfactant-BL was only 18.8%, despite the initially unfavorable prognosis.
BREATHE EASILY!